




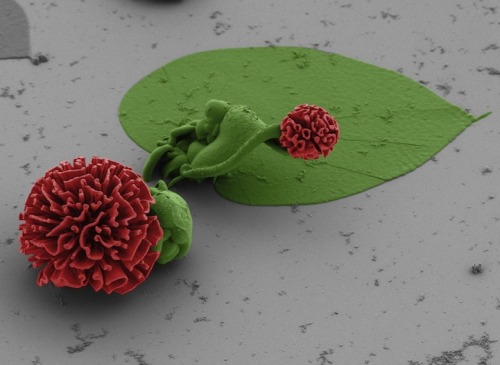

Microscopic flowers by Harvard School of Engineering and Applied Sciences (SEAS) post-doctoral fellow Wim L. Noorduin.
About the project:
About the project:
To create the flower structures Noorduin and his colleagues dissolve barium chloride (a salt) and sodium silicate (also known as waterglass) into a beaker of water. Carbon dioxide from air naturally dissolves in the water, setting off a reaction which precipitates barium carbonate crystals. As a byproduct, it also lowers the pH of the solution immediately surrounding the crystals, which then triggers a reaction with the dissolved waterglass. This second reaction adds a layer of silica to the growing structures, uses up the acid from the solution, and allows the formation of barium carbonate crystals to continue. Stacey Thinx